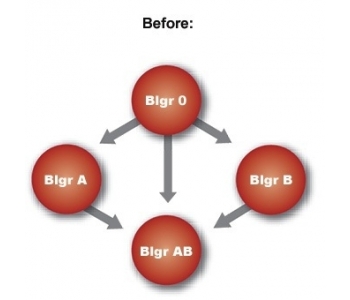
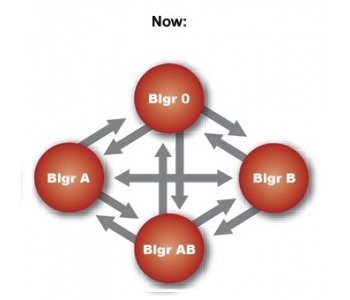
Anti-A and/or anti-B antibodies cause the ABO-incompatibility. These antibodies constitute less than 1% of a patient's total amount of antibodies. Over 15 years, clinical experience has shown that ABO-incompatible transplants ca be safely performed after treatment with Glycosorb®-ABO. Short and long term results are excellent.
Glycosorb®-ABO is a medical device based on biologically active carbohydrates (blood group A and B antigens), to which anti-A/B antibodies specifically bind, and are thus removed from the patient's plasma.
- Safe and effective treatment of several patient plasma volumes in one treatment
- Other antibodies and blood components are not affected
- No replacement fluids are required
- Glycosorb®-ABO is steam-sterilized and free from any toxi chenicals and preservatives.
Anti-A/B antibody specific removal
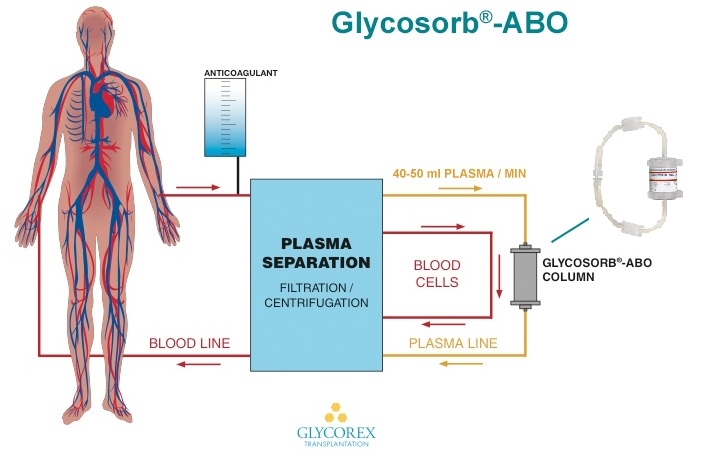
The treatment with Glycosorb®-ABO can be carried out at any facilities qualified for the performance of apheresis. Glycosorb®-ABO is compatible with a wide range of plasma separators, both centrifugation and filtration equipments. The duration of a treatment session usually ranges between 1-6 hours and depends on the size of the patient and the total number of treated plasma volumes.
As in other extracorporeal procedures, an anti-coagulant is administered. Plasma is separated from the blood cells and is passed through Glycosorb®-ABO. It specifically binds the anti-A/B antibodies in the plasma, and the treated plasma is reunited with the blood cells and returned to the patient in a closed circuit. No replacement fluids are required.
The anti-A/B antibody titer is normally reduced to 1:1 or less when measure in blood plasma directly after passage through Glycosorb®-ABO, even in Blood plasma with high titers. (A target anti-A/B antibody titer of 1:4 or less on the day of transplant is recommended to minimized the ridk of anti-A/B antibody mediated rejection.